Our genes shape most of what we look like and how our bodies work – the very fabric of our being. Humanity’s understanding of genetics has come a long way since Gregor Mendel, the Father of Genetics, carried out his fateful experiment on peas in 1865. Genes have been found to be intricately linked to our individual likelihoods of developing certain diseases.
A famous example is the BRCA1 gene that imparts a 30-60% risk of developing breast cancer. It was the presence of this gene that spurred superstar Angelina Jolie to prophylactically remove both breasts in efforts of eliminating her chances of ever developing breast cancer.
With that, we take a look at the bug that took the world by storm – COVID-19. The numbers are staggering – as of 10th August, 2021, there have been over 200million cases of COVID-19 around the world, with 4 million deaths.
The numbers are staggering – as of 10th August, 2021, there have been over 200million cases of COVID-19 around the world, with 4 million deaths.
In this article, we discuss current evidence on your genetic risk of COVID-19. How do genes alter your body’s response to COVID-19? What do MTHFR genes have to do with COVID? How are increased homocysteine levels related to COVID-19 infections? Read on to learn more.
Genetics and viral infections
Viral infections are sources of evolutionary pressures, causing the human biology to adapt over time. Call it a form of ‘natural selection’, if you will. As a result, several gene variations (called gene polymorphisms) that either increase or decrease the likelihood of viral disease have been documented in influenza virus (the common cold), human immunodeficiency virus (HIV), human T-cell leukemia virus type 1 (HTLV-1, which causes T-cell leukemia or lymphoma), hepatitis C and B, as well as herpes virus, to name a few (1).
Minor gene polymorphisms change how our body responds to infections by affecting how the virus enters cells in our bodies (virus receptors) and how our immune system detects and reacts to foreign viral invasions. Figure 1 outlines the many ways that genes can change our resistance or predisposition to viral diseases.
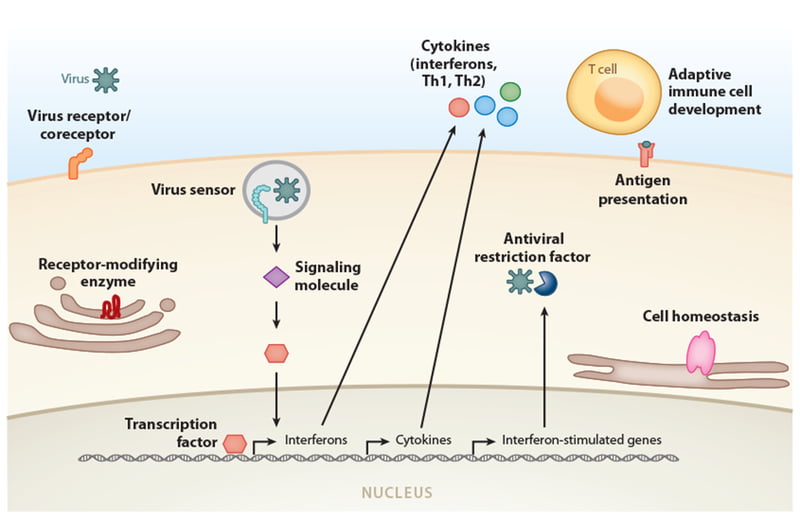
Figure 1: Categories of genes where variants have been associated with susceptibility or resistance to specific viral diseases.
Image from: Kenney AD, Dowdle JA, Bozzacco L, et al. Human Genetic Determinants of Viral Diseases. Annu Rev Genet. 2017;51:241-263. doi:10.1146/annurev-genet-120116-023425
Genetic susceptibility to COVID-19
COVID-19 is caused by the SARS-CoV-2, a coronavirus. Besides the lungs, COVID-19 has the potential to attack nearly every other organ in the body (Figure 2). You may have heard of some families where everyone ends up needing intensive care for COVID-19, while some breeze through quarantine with nothing but a cough and some runny nose. Could these patterns be due to genetics?
We know that COVID-19 hits hard in the elderly,
We know that COVID-19 hits hard in the elderly, and those with weak immune systems like cancer, autoimmune disease, diabetes, chronic kidney disease, and lung disease. In efforts to look for genetic risk factors, scientists from around the world have pooled their resources into forming the COVID-19 Host Genetics Initiative. This massive human genetic study on COVID-19 infections have already identified 13 different locations in the human genome that may affect your likelihood of getting the infection, as well as the severity of disease if you get it (2).
What are the gene polymorphisms involved in COVID-19 risk?
Intense research has brought forth several theories regarding the genetic underpinnings of how the body reacts to COVID-19 infection. The first of these is genes affecting the angiotensin-converting enzyme 2 (ACE-2) receptor, which is how the SARS-CoV-2 virus enters the human cell. Polymorphisms here may make it easier, or harder, for the virus to gain a foothold into our system (3).
Secondly, genetic variation in components of the immune system, namely the human leukocyte antigen (HLA) system, may influence susceptibility to COVID-19. Genetic differences in the HLA molecule has long been associated with varying immune responses against pathogens, and an individuals risk of developing autoimmune disease (3).
COVID-19 does the most damage to the body by causing a ‘cytokine storm’ – a relentless cascade of proinflammatory cytokine release that causes rapid clinical deterioration. Genes that affect the production and amount of cytokines are therefore able to influence COVID-19 severity (3). Given the prominent role of inflammation in COVID-19, we move on to the MTHFR gene as a key modulator of B vitamins and homocysteine, both of which are prominent inflammation regulators in the body.
MTHFR polymorphisms and COVID-19
The MTHFR gene, short for methylenetetrahydrofolate reductase, is responsible for producing the MTHFR gene in the body. MTHFR dictates the levels of two B vitamins in your body – folate (vitamin B9) and cobalamin (vitamin B12). Both these vitamins are needed for a healthy immune system, new DNA production, and cell growth. Low levels of these vitamins cause pancytopenia (reduced red and white blood cell counts and platelets), and cognitive changes (fatigue, irritability, depression).
Homocysteine is another molecule that is related to the MTHFR enzyme, which usually converts excess homocysteine into methionine. Without MTHFR, homocysteine builds up in the body, leading to deleterious effects in the heart, blood vessels, brain, nerves, and bones.
Extremely high homocysteine levels increase the chances of the body forming a dangerous blood clot.
Low MTHFR enzyme activity leads to both low folate, low cobalamin, and high homocysteine levels (4). One way that leads to intrinsically low MTHFR enzyme activity is the presence of certain MTHFR polymorphisms that alter the amount of functional MTHFR enzyme that the body produces. The two most common polymorphisms are the C677T and A1298C mutations (5).
Interestingly, a study that compared the distributions of the C677T MTHFR polymorphism against COVID-19 incidence and mortality rates found that countries with the highest number of people with the C677T polymorphism (Latino and European countries) also had the highest numbers of recorded COVID19 cases and deaths (6).
‘Long COVID’ – a debilitating aftermath of acute COVID-19 infection
Before we piece together how folate, cobalamin, and homocysteine are involved in COVID-19, let’s talk about ‘long COVID’, also known as post-acute COVID-19 syndrome.
While fortunately a large proportion of patients breathe a sigh of relief after recovering from COVID-19, there are some who will be struggling to breathe for much longer. ‘Long COVID’ are persistent symptoms beyond 4 – 12 weeks after acute COVID, causing feelings of fatigue, difficulty concentrating (brain fog), and a myriad of symptoms such as breathlessness, cough, anxiety, depression, chest pain, chronic kidney disease, and thromboembolisms (7).
causing feelings of fatigue, difficulty concentrating (brain fog), and a myriad of symptoms such as breathlessness, cough, anxiety, depression, chest pain, chronic kidney disease, and thromboembolisms (7).
An Italian study reported up to 87% of 143 patients experiencing persistent symptoms nearly 2 months after acute COVID (8).
Researchers have drawn several parallels between the symptoms of long COVID and vitamin B12 deficient diseases like pernicious anemia and myalgic encephalitis/chronic fatigue syndrome (9). Could an imbalance of folate, cobalamin, and homocysteine be responsible for the debilitating sequelae of COVID?
COVID-19 – A methyl group assault?
Have a look at Figure 2. We see that homocysteine, folate, and cobalamin are linked by the MTHFR enzyme in processes collectively referred to as the one-carbon metabolism (9).
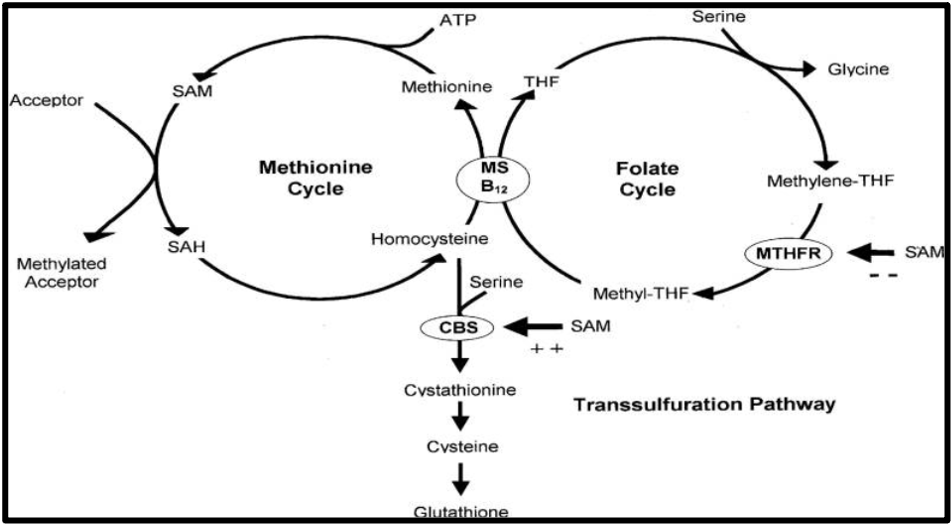
Figure 2: The one-carbon metabolism pathway
ATP, adenosine triphosphate; CBS, cystathionine beta synthase; MS, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; THF, tetrahydrofolate
In the methionine cycle, pay attention to the SAM molecule, which acts as a universal methyl donor. SAM loses its methyl group, forming SAH, which becomes homocysteine and eventually methionine again.
How does COVID-19 fit into all this? Well, the SARS-CoV-2 virus is thought to interfere with the one-carbon metabolism by:
- Reducing SAM levels
- Using up methyl groups for viral replication
By simultaneously increasing demand and depleting supply of methyl groups, COVID-19 wreaks havoc with the body’s one-carbon metabolism pathways, ultimately leading to imbalanced folate, cobalamin, and homocysteine levels. As we’ll soon discuss, altered amounts of these nutrients may potentially influence how a person reacts to an infection.
Increased homocysteine levels and COVID-19
Doctors discovered early on that COVID-19 infections are notorious for causing blood clots throughout the body. They may occur in the brain (ischemic stroke), legs (deep vein thrombosis, DVT), and lungs (pulmonary embolism, PE). A meta-analysis on 102 studies including 64,503 patients with COVID-19 found that PE and DVT occurred at rates of 8% and 11%, respectively. COVID patients who needed intensive care had a 23% of developing any venous thromboembolism (which included PE and DVT) (10).
A meta-analysis on 102 studies including 64,503 patients with COVID-19 found that PE and DVT occurred at rates of 8% and 11%, respectively.
A number of studies suggest that high homocysteine levels put you at risk of getting blood clots (11). It remains plausible that having an MTHFR polymorphism that increases homocysteine levels could result in more severe COVID-19 infection, simply by raising the overall risks of a blood clot. What’s more, two MTHFR polymorphisms, A222V and E429A, were found to alter homocysteine levels (12).
Elevated homocysteine levels are also predictive of COVID-19 disease progression. In a study of 273 patients with mild COVID-19, homocysteine levels of more than 15.4 µmol/L had a three-fold increased risk of disease progression as evidenced by worsened CT lung images (13).
Low folate and cobalamin in COVID-19
Evidence is scarce on the direct relationship between B vitamins and COVID-19 infections. Nevertheless, there are no studies that show that being folate deficient results in worse COVID-19 clinical outcomes.
While a study on 333 patients hospitalised for COVID-19 found that 11% of them were folate deficient,
While a study on 333 patients hospitalised for COVID-19 found that 11% of them were folate deficient, researchers did not find any significant association between low folate levels and poor clinical outcomes (14).
On the other hand, a small clinical trial found that giving hospitalised COVID-19 patients aged 50 and above a combination of vitamin D, magnesium, and vitamin B12 supplements appeared to reduce the risk of needing oxygen or intensive care support (15). The authors postulate that these benefits may be due to vitamin D’s anti-inflammatory properties, magnesium’s role as a cofactor in vitamin D activation, and vitamin B12 as an essential immune system supporter.
Keeping homocysteine low and B vitamins high
The battle against COVID-19 looks to be a long one, and we need all the help we can get.
Your next steps are:
- Evaluate your risk – inflammation, Vitamin D, homocysteine, Vitamin B12 and folate levels
- Prepare for vaccination and support post vaccination if you decide to get it
- Provide antiviral and immune support on an ongoing basis
If you would like to find out more here are some resources to assist you:
Here’s a free snapshot from our recent COVID-19 Update webinar
To watch the full webinar – join our Patient Knowledge Centre. You will also receive each and every webinar for FREE, including all our resources, Facebook sessions to support you and your family.
Or Purchase the webinar here for $39.
Or make an appointment with one of our practitioners here.
References
1.Kenney AD, Dowdle JA, Bozzacco L, McMichael TM, St Gelais C, Panfil AR, et al. Human Genetic Determinants of Viral Diseases. Annu Rev Genet. 2017;51:241-63.
2.Initiative C-HG. Mapping the human genetic architecture of COVID-19. Nature. 2021.
3.Debnath M, Banerjee M, Berk M. Genetic gateways to COVID-19 infection: Implications for risk, severity, and outcomes. The FASEB Journal. 2020;34(7):8787-95.
4.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34(1):75-81.
5.Levin BL, Varga E. MTHFR: Addressing Genetic Counseling Dilemmas Using Evidence-Based Literature. J Genet Couns. 2016;25(5):901-11.
6.Ponti G, Pastorino L, Manfredini M, Ozben T, Oliva G, Kaleci S, et al. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J Clin Lab Anal. 2021;35(7):e23798-e.
7.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27(4):601-15.
8.Carfì A, Bernabei R, Landi F, Group ftGAC-P-ACS. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603-5.
9.McCaddon A, Regland B. COVID-19: A methyl-group assault? Med Hypotheses. 2021;149:110543-.
10.Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021:thoraxjnl-2020-215383.
11.Abu-Farha M, Al-Sabah S, Hammad MM, Hebbar P, Channanath AM, John SE, et al. Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A Focused Analysis on D-Dimer, Homocysteine and Thromboembolism. Frontiers in Pharmacology. 2020;11(1960).
12.Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
13.Yang Z, Shi J, He Z, Lü Y, Xu Q, Ye C, et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging (Albany NY). 2020;12(7):6037-48.
14.Meisel E, Efros O, Bleier J, Beit Halevi T, Segal G, Rahav G, et al. Folate Levels in Patients Hospitalized with Coronavirus Disease 2019. Nutrients. 2021;13(3):812.
15.Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition. 2020;79-80:111017-.