You say folate, I say folic acid… let’s call the whole thing off? Paraphrasing the famous lyrics may help us remember that similar sounding words may not always translate to the same molecules!
Folate has become an umbrella term used interchangeably to describe different forms of the basic vitamin B9: it can be easy to get lost in the various important issues that surround right choice, current health status and what you are trying to achieve with regards to your health. This blog entry will try to clarify this by looking at the chemical structure, how it gets modified in the body and how it is absorbed.
Natural source vs. Supplementation
Quite simply, folate applies to naturally occurring molecules. When eating dark leafy greens, some types of beans or even beef liver, folates are ingested. Folic acid applies to the form that comes from a plastic bottle such as a multivitamin, any other tablet formulation, or eating food that has been intentionally supplemented with the synthetic molecule.
The natural source folates are chemically unstable
Have you ever wondered why recommended amounts of vitamins and/or vitamin-rich food stuffs are often given as a range, such as 1-2 pieces of fruit? One of the reasons for that is related to how long a molecule retains its original shape or structure. The naturally occurring folates in foodstuffs are chemically unstable. Naturally occurring dietary folates are all based on the structure in Figure 1. Folates are reduced (see the hydrogen H attached to the nitrogen N at the left) and they carry different numbers of glutamate molecules on their “tail” (see the Glu tail at right). The linkage between the pterin portion and the pABA portion in natural folates is particularly sensitive to falling apart: once split into two, it become easy to see that neither part of the molecule has biologic activity. Other sections of www.mthfrsupport.com.au explain how the MTHFR enzyme needs to bind to the whole folate to produce the active folate 5-MTHFR that is so critical to many biochemical processes.
Figure 1:
Different foods contain different forms of folates, and the take-home message is that reduced folates with shorter or longer chains of glutamate are less stable molecules: natural folates rapidly lose activity in foods over periods of days or weeks, but there is still enough that makes it through the digestive system to have a beneficial effect on health.
Folic acid used in supplementation is chemically stable
In contrast to natural folates, folic acid used for supplementation either in foodstuffs, or as an oral supplement, is almost completely stable for months or even years. Notice how overall, the structure in Figure 2 is quite similar to the structure of Figure 1: the important difference if the absence of hydrogen H attached to the nitrogen N in the pteridine (a.k.a. pterin) ring on the left. This is referred to as the oxidized form. Folic acid also carries only one glutamate molecule.
The reason for the simplicity of the structure synthetic molecule is just that…because it is synthetic: although synthesis in the laboratory can be quite powerful even in 1943 (the year folic acid was first produced), achieving the complexity of structures we see in nature is really the specialty of Mother Nature, even in the 21st century laboratory.
Figure 2: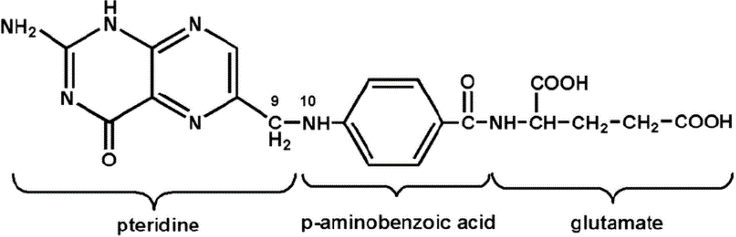
So what’s next for folates and folic acid? Once foodstuffs full of folates and folic acid make it through the stomach and into the gut, a few important steps happen.
Absorption into cells and the bloodstream.
As we mentioned earlier, a lot of the dietary folates survive long enough to make it into the gut. Once there, the folate glutamate “tails” must be removed by an intestinal brush border membrane glutamylhydrolase enzyme (glutamate carboxypeptidase II, GCPII) in the small intestine to leave behind a single (“mono”) glutamate (as in Figure 2) before absorption across the intestinal mucosa. Folic acid already carries a single glutamate, so it is not processed by this enzyme. Absorption of the monoglutamate form can occur via a protein which carries it across cell membranes, or if there is such a large amount in the gut, it can simply diffuse across the cell membranes.
What really matters at this point is that all monoglutamate forms are then channeled to enter the pathways leading to the 5-methyltetrahydrofolate (5-methyl-THF) form that will enter the bloodstream.
Bioavailability is the next concept that, depending on your perspective, either unites dietary folates and synthetic folic acid, or informs your decision-making to fit your current needs and lifestyle.
Bioavailability of folates and folic acid dictates how much you actually absorb into your blood.
The Oxford Dictionary of Diet and Exercise defines bioavailability as the quantity or fraction of material ingested by mouth that is absorbed into the bloodstream. The bioavailability of dietary folates can vary not only based on the chemical stability discussed earlier, but whether the dietary folates can actually be extracted from the foodstuffs ingested. For example, some dietary folates may be “trapped” in the cellular structure or insoluble matrix of certain foods; the natural acidity of the stomach may destroy some of the dietary folates; some of the glutamate “tails” may not all be efficiently removed, precluding passage across the intestinal mucosa. These issues are considered negligible in the case of folic acid. When ingested as a tablet or as an additive to foodstuffs, the extraction from cellular structures is no longer an issue; the oxidized form discussed in Figure 2 is chemically more stable and so less susceptible to destruction within the digestive system; the monoglutamate form is ready for subsequent enzymatic modification.
With a better understanding of the structural difference between folates and folic acid, and the concept of bioavailability, it is easy to see that conversations with your physician or nutritionist become very important in deciding what is best for you.
Crécy-Lagard, V., El Yacoubi, B., Diaz de la Garza, R.,Noiriel, A. & Hanson, A. (2007). Comparative genomics of bacterial and plant folate synthesis and salvage: Predictions and validations. BMC genomics. 8. 245.
Doi:10.1186/1471-2164-8-245.
Caudill MA.(2010) Folate bioavailability: implications for establishing dietary recommendations and optimizing status. The American Journal of Clinical Nutrition. 91(5):1455S-1460S. Doi:10.3945/ajcn.2010.28674E.
Mathias, R., Ribeiro, P.R.S., Sarraguça, M.C. & Lopes, J.A. (2014). A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Anal. Methods, 6, 3065-3071.
Doi:10.1039/C3AY41874J
Report of a joint FAO/WHO expert consultation Bangkok, Thailand (2001) Food and Agriculture Organization of the United Nations World Health Organization